7th Gene Therapy for CNS Summit – What’s it About?
The 7th Gene Therapy for CNS Summit is set to unite key leaders across the gene therapy space, focused on targeting the CNS to treat important neurodegenerative disorders. You can expect to meet C-Level Leaders, Directors, Vice Presidents, Heads, and Scientists all within discovery, research, translational development, clinical development, and regulatory affairs.
This is a must-attend meeting to ensure you are networking with like-minded colleagues facing the same daily challenges surrounding vector development, BBB crossing, identifying accurate CNS models and more – to jointly overcome them and move the needle forward on gene therapy development.
Key Focus Areas of This Year’s Agenda:
Download the Brochure to Explore Sessions Covering the Focus Areas Outlined Above

Stay Ahead of the Curve in CNS Gene Therapy Innovation
With recent groundbreaking advancements shaking up the CNS gene therapy space, now is the time to join leading experts shaping the future of targeted delivery.
- FDA Approves First Brain-Delivered AAV Gene Therapy
In a historic first, the FDA has approved an AAV gene therapy directly delivered to the brain, marking a major milestone for neuro-focused treatments. - Capsida Secures IND Clearance for IV-Delivered CNS Gene Therapy
Capsida has received FDA IND clearance for its first-in-class intravenously administered gene therapy for STXBP1-related epileptic encephalopathy, underscoring the growing focus on targeted, non-invasive delivery approaches. - Dyno Therapeutics Launches Breakthrough Capsids for CNS, Eye & Muscle
Dyno Therapeutics has unveiled three next-gen capsid delivery vectors, promising to enhance precision and efficiency for CNS-targeted therapies.
⭐ Don’t miss insights from Eric Kelsic, CEO & Co-founder of Dyno, speaking live at the meeting. Read more › - Sangamo and Lilly Sign CNS Capsid Licensing Deal
In another signal of accelerating progress, Sangamo Therapeutics has entered a licensing agreement with Eli Lilly to advance novel CNS-targeted capsids.
⭐ Gain first-hand insights from Mohammad Samie, Associate Director, Program Leadership at Sangamo, who will be speaking at the event. Read more ›
Who Will You Meet?
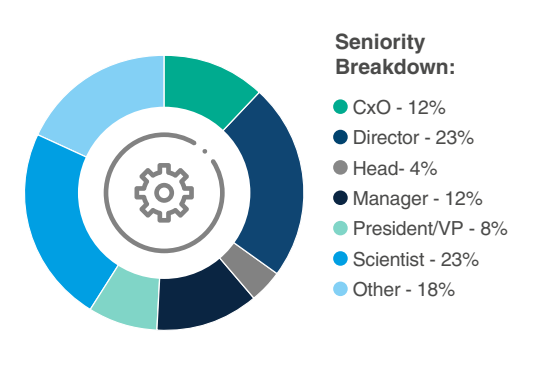
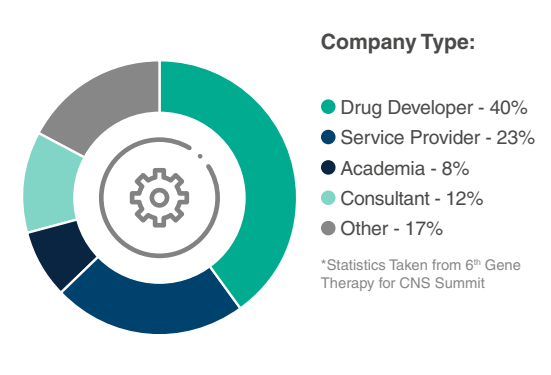
Have more specific questions about attending companies or their sessions?
Download the full brochure here or email us directly at info@hansonwade.com — we’re happy to help!







