Be at the Forefront of CNS Gene Therapy Progress
In 2025, we will unite over 100 drug developers working in CNS-directed gene therapies from leading biopharma, and we anticipate the meeting will grow even further this year.
Neurological disorders will continue to be the most investigated disease area in the gene therapy field, with more drugs than ever expected to progress from preclinical stages into Phase 1 trials.
This conference will offer the perfect platform to showcase your brand, expertise, and reputation to those driving development in 2025.
Experts Need Your Help
Backed by in-depth research with CNS gene therapy developers, we uncover exactly what biopharma is looking for in solution partners - offering you a unique opportunity to align your services with the critical needs driving faster, smarter development.
In Vivo & In Vitro Preclinical Models
The translatability of preclinical models when scaling up from an in vivo model to a human brain remains a key challenge in neurology. Help drug developers overcome the limited availability of preclinical models to progress their assets safely to in-human clinical trials and through those all-important regulatory milestones
Drug Delivery Technology
To deliver their therapeutics with maximum efficacy and minimal safety risks, drug developers need the best capsid design and direct brain and CSF delivery. Showcase your ground-breaking technologies to ensure the success of CNS direct gene therapies
Diagnostic Tools & Digital Biomarkers
Early disease intervention is especially challenging in neurological disorders and tracking this disease progression can be even more difficult. Developers are seeking for the latest and greatest imaging and wearable monitoring technology to overcome these roadblocks. Highlight your innovative solutions for clinical monitoring, providing the depth and breadth of data needed for successful regulatory approval
CROs
Clinical trial management, patient recruitment and data reporting are key to successfully advancing clinical programs, positive regulatory meetings, and strong market access. Demonstrate how your CRO services can empower CNS-directed gene therapy developers to achieve these critical milestones
Why Partner?
Meet your 2025 commercial objectives and educate decision-makers on how your expertise can overcome immunogenicity bottlenecks in gene therapy development
Showcase your expertise to leading gene therapy organizations by securing an exhibition booth to set yourself as the go-to service provider in this community
Demonstrate thought leadership and drive brand exposure by securing a speaking slot on the agenda to connect with the community and generate partnerships
Maximize the balance of content and networking to generate leads and build new relationships with industry pioneers
Who Will You Meet?
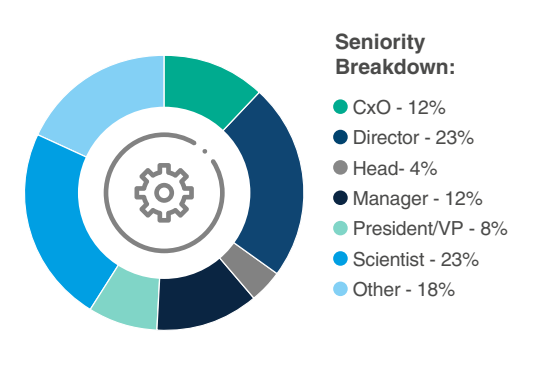
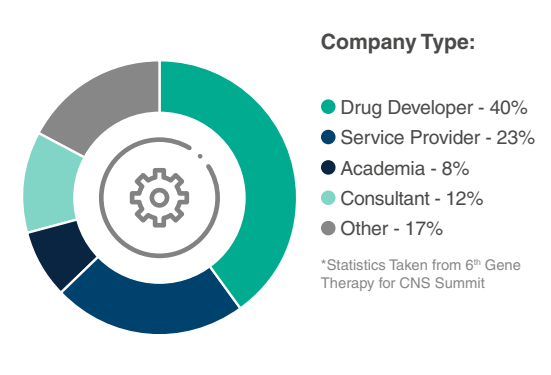
Have more specific questions about our attendees?
Download the full brochure here or email us directly at sponsor@hansonwade.com - we’re happy to help!
Explore Other Related Events
The Gene Therapy Event Series spans in-depth content for each role and specification in gene therapy drug development, so you can curate your event calendar to meet industry leaders most relevant to your area of expertise across the year. Download our Partnership Prospectus now to find out more.

